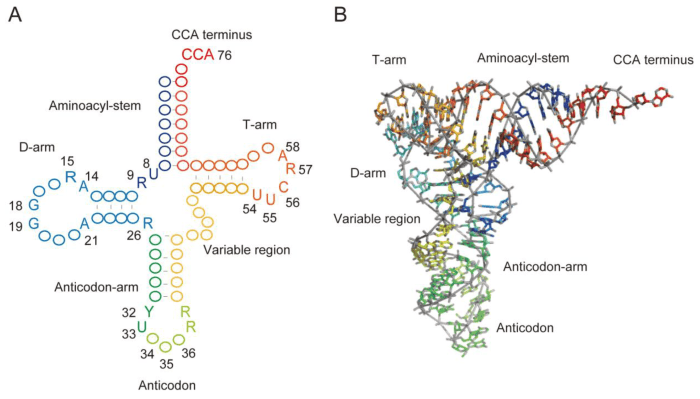
Label the structural features of the yeast phenylalanine trna. – Labeling the structural features of the yeast phenylalanine tRNA is a crucial step in understanding the intricate mechanisms of protein synthesis. This molecule, with its unique characteristics, plays a pivotal role in deciphering the genetic code and orchestrating the assembly of amino acids into functional proteins.
The cloverleaf model of tRNA, with its distinct anticodon loop, provides a foundation for exploring the structural intricacies of yeast phenylalanine tRNA. Delving deeper into the tertiary structure reveals the complex interactions that stabilize the molecule and facilitate its interactions with other components of the translation machinery.
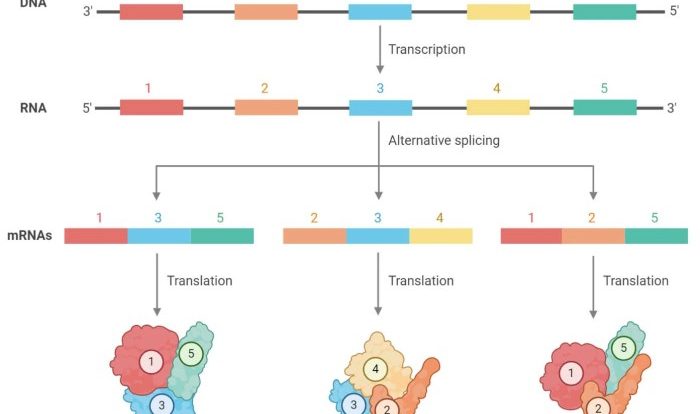
tRNA Structure
tRNA molecules are essential components of the protein synthesis machinery. They are responsible for transferring amino acids to the growing polypeptide chain during translation. tRNA molecules have a characteristic cloverleaf structure, which consists of four loops: the acceptor stem, the anticodon stem, the D-stem, and the T-stem.
Cloverleaf Model of tRNA
The cloverleaf model of tRNA is a two-dimensional representation of the molecule. The acceptor stem is located at the bottom of the cloverleaf, and it contains the site where the amino acid is attached. The anticodon stem is located at the top of the cloverleaf, and it contains the anticodon, which is a three-nucleotide sequence that is complementary to the codon on the mRNA.
Anticodon Loop
The anticodon loop is a seven-nucleotide loop that is located at the end of the anticodon stem. The anticodon loop interacts with the mRNA codon through complementary base pairing. This interaction is essential for ensuring that the correct amino acid is incorporated into the growing polypeptide chain.
Yeast Phenylalanine tRNA
Yeast phenylalanine tRNA is a specific type of tRNA that is responsible for transferring the amino acid phenylalanine to the growing polypeptide chain. Yeast phenylalanine tRNA has a unique structural feature that distinguishes it from other tRNA molecules: it contains a modified nucleotide called wybutosine in the anticodon loop.
Unique Characteristics
Wybutosine is a modified guanine nucleotide that is found only in yeast phenylalanine tRNA. It is thought to play a role in the stability of the tRNA molecule and in its ability to interact with the mRNA codon.
Comparison to Other tRNA Molecules
Yeast phenylalanine tRNA is similar in structure to other tRNA molecules, but it has a few unique features that distinguish it. These features include the presence of wybutosine in the anticodon loop and a slightly different sequence in the D-stem.
Anticodon Loop
The anticodon loop of yeast phenylalanine tRNA is a seven-nucleotide loop that is located at the end of the anticodon stem. The anticodon loop contains the anticodon, which is a three-nucleotide sequence that is complementary to the codon on the mRNA.
Sequence and Structure
The anticodon of yeast phenylalanine tRNA is GAA, which is complementary to the codon UUC on the mRNA. The anticodon loop is stabilized by a number of hydrogen bonds, including two base pairs between the anticodon and the mRNA codon.
Interaction with mRNA Codon
The anticodon loop of yeast phenylalanine tRNA interacts with the mRNA codon through complementary base pairing. This interaction is essential for ensuring that the correct amino acid is incorporated into the growing polypeptide chain.
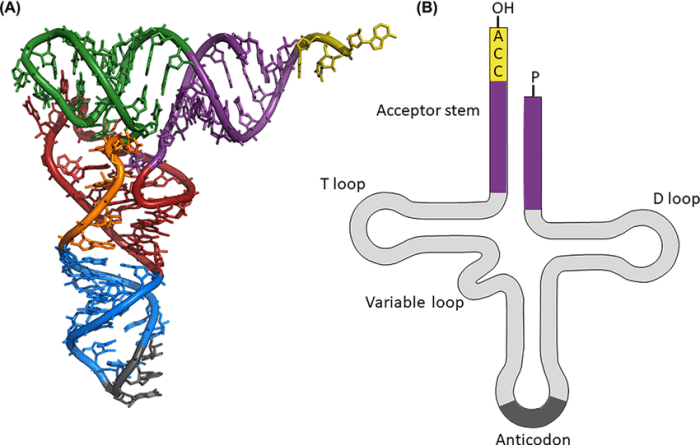
Tertiary Structure: Label The Structural Features Of The Yeast Phenylalanine Trna.
The tertiary structure of yeast phenylalanine tRNA is a three-dimensional structure that is formed by the folding of the cloverleaf structure. The tertiary structure is stabilized by a number of interactions, including hydrogen bonds, van der Waals forces, and hydrophobic interactions.
Cloverleaf Model and Tertiary Structure
The cloverleaf model of tRNA is a two-dimensional representation of the molecule, while the tertiary structure is a three-dimensional representation. The tertiary structure is more compact than the cloverleaf structure, and it is more closely related to the actual structure of the tRNA molecule.
Interactions Stabilizing Tertiary Structure
The tertiary structure of yeast phenylalanine tRNA is stabilized by a number of interactions, including hydrogen bonds, van der Waals forces, and hydrophobic interactions. Hydrogen bonds are formed between the hydrogen atoms of one molecule and the oxygen or nitrogen atoms of another molecule.
Van der Waals forces are weak attractive forces that occur between all molecules. Hydrophobic interactions are attractive forces that occur between nonpolar molecules.
Chemical Modifications
Yeast phenylalanine tRNA contains a number of chemical modifications that are not found in other tRNA molecules. These modifications include the presence of wybutosine in the anticodon loop and a number of other modified nucleotides.
Role in tRNA Stability and Function
The chemical modifications in yeast phenylalanine tRNA play a role in the stability and function of the molecule. Wybutosine is thought to stabilize the tRNA molecule and to help it interact with the mRNA codon. Other modified nucleotides may also play a role in the stability and function of the tRNA molecule.
Unique Properties of Yeast Phenylalanine tRNA
The chemical modifications in yeast phenylalanine tRNA contribute to the unique properties of the molecule. These modifications make the tRNA molecule more stable and more efficient at interacting with the mRNA codon.
Functional Significance
The structural features of yeast phenylalanine tRNA are essential for its function in protein synthesis. The cloverleaf structure allows the tRNA molecule to fold into a compact and stable conformation. The anticodon loop allows the tRNA molecule to interact with the mRNA codon.
The tertiary structure further stabilizes the tRNA molecule and helps it to interact with other components of the protein synthesis machinery.
Accurate and Efficient Translation, Label the structural features of the yeast phenylalanine trna.
The structural features of yeast phenylalanine tRNA contribute to the accurate and efficient translation of the genetic code. The cloverleaf structure allows the tRNA molecule to fold into a compact and stable conformation, which is essential for its interaction with other components of the protein synthesis machinery.
The anticodon loop allows the tRNA molecule to interact with the mRNA codon, which is essential for ensuring that the correct amino acid is incorporated into the growing polypeptide chain.
Implications of Structural Variations
Structural variations in yeast phenylalanine tRNA can have implications for protein synthesis. Mutations in the tRNA molecule can affect its stability, its ability to interact with the mRNA codon, or its ability to interact with other components of the protein synthesis machinery.
These mutations can lead to errors in protein synthesis, which can have a variety of consequences for the cell.
Essential Questionnaire
What is the significance of the anticodon loop in yeast phenylalanine tRNA?
The anticodon loop is crucial for protein synthesis as it interacts with the mRNA codon during translation. This interaction ensures the correct amino acid is incorporated into the growing polypeptide chain.
How does the tertiary structure of yeast phenylalanine tRNA contribute to its function?
The tertiary structure stabilizes the tRNA molecule and facilitates its interactions with other components of the translation machinery. This precise arrangement enables the tRNA to efficiently deliver its amino acid cargo to the ribosome.
What are the implications of structural variations in yeast phenylalanine tRNA?
Structural variations in yeast phenylalanine tRNA can affect its ability to bind to the mRNA codon and interact with other translation factors. These variations can impact protein synthesis accuracy and efficiency, potentially leading to cellular dysfunction.