Determine the type of alcohol represented by each structure. – Determine the type of alcohol represented by each structure, delving into the fascinating realm of organic chemistry. By examining the intricate molecular structures of alcohols, we unravel the secrets behind their diverse properties and behavior.
This exploration unveils the fundamental building blocks of alcohols, deciphering the language of functional groups and their profound impact on alcohol classification. We embark on a journey to discern primary, secondary, and tertiary alcohols, guided by the number of carbon atoms embracing the hydroxyl group.
Introduction to Alcohol Structures
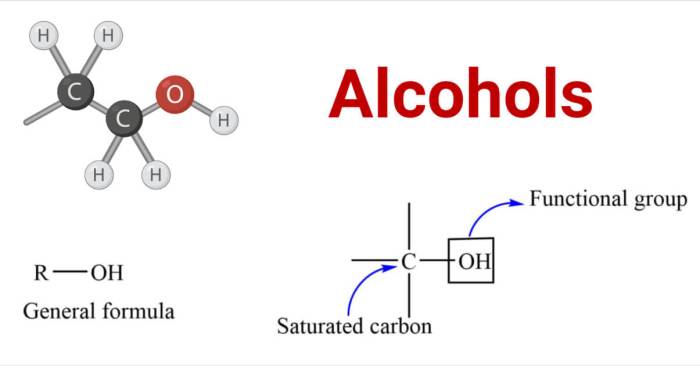
Alcohols are organic compounds that contain a hydroxyl group (-OH) bonded to a carbon atom. The fundamental building blocks of alcohol structures include:
- Carbon atoms
- Hydrogen atoms
- Oxygen atom
Alcohols can also contain other functional groups, such as:
- Alkenes (C=C double bond)
- Alkynes (C≡C triple bond)
- Aromatic rings
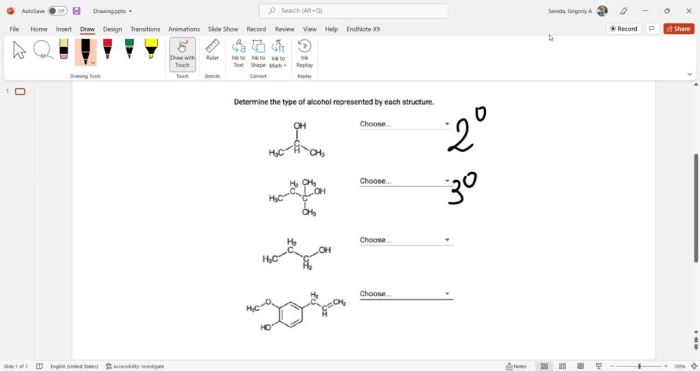
Determining Alcohol Type from Structure
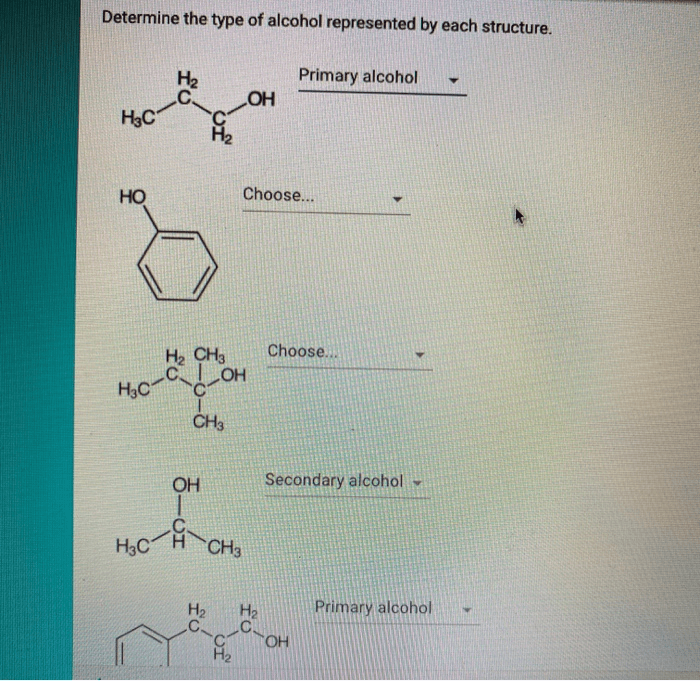
The type of alcohol (primary, secondary, or tertiary) is determined by the number of carbon atoms bonded to the carbon atom bearing the hydroxyl group:
- Primary alcohol:The carbon atom bearing the hydroxyl group is bonded to one other carbon atom.
- Secondary alcohol:The carbon atom bearing the hydroxyl group is bonded to two other carbon atoms.
- Tertiary alcohol:The carbon atom bearing the hydroxyl group is bonded to three other carbon atoms.
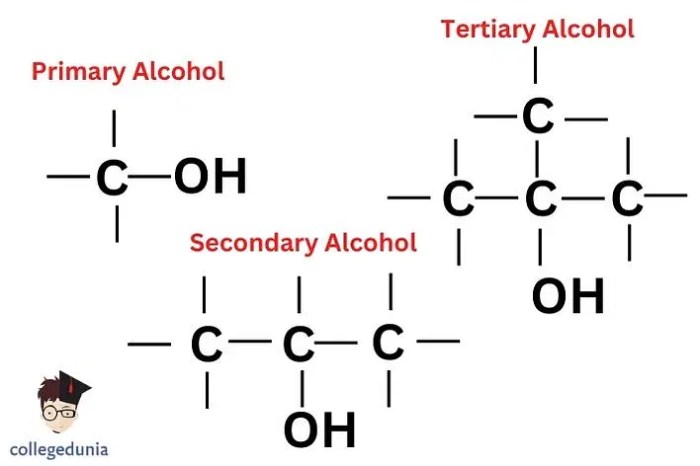
The structural characteristics that distinguish primary, secondary, and tertiary alcohols are:
- Primary alcohol:R-CH 2-OH
- Secondary alcohol:R 2CH-OH
- Tertiary alcohol:R 3C-OH
Examples of Alcohol Structures
| Classification | Structural Formula | IUPAC Name |
|---|---|---|
| Primary alcohol | CH3CH2OH | Ethanol |
| Secondary alcohol | (CH3)2CHOH | 2-Propanol |
| Tertiary alcohol | (CH3)3COH | 2-Methyl-2-propanol |
| Cyclic alcohol | C6H11OH | Cyclohexanol |
Impact of Structure on Alcohol Properties
The type of alcohol (primary, secondary, tertiary) influences its physical and chemical properties:
- Boiling point:Primary alcohols have higher boiling points than secondary alcohols, which have higher boiling points than tertiary alcohols.
- Solubility:Primary alcohols are more soluble in water than secondary alcohols, which are more soluble in water than tertiary alcohols.
- Reactivity:Tertiary alcohols are more reactive than secondary alcohols, which are more reactive than primary alcohols.
Advanced Analysis of Alcohol Structures: Determine The Type Of Alcohol Represented By Each Structure.
The hybridization of the carbon atom bonded to the hydroxyl group can be determined by:
- sp3hybridization: The carbon atom is bonded to four other atoms, forming a tetrahedral geometry.
- sp2hybridization: The carbon atom is bonded to three other atoms, forming a trigonal planar geometry.
- sp hybridization:The carbon atom is bonded to two other atoms, forming a linear geometry.
The hybridization of the carbon atom bonded to the hydroxyl group has implications for alcohol reactivity and behavior:
- sp3hybridization: The carbon atom is less reactive and more stable.
- sp2hybridization: The carbon atom is more reactive and less stable.
- sp hybridization:The carbon atom is the most reactive and least stable.
Query Resolution
What are the key structural features that distinguish primary, secondary, and tertiary alcohols?
Primary alcohols have one carbon atom bonded to the hydroxyl group, secondary alcohols have two carbon atoms bonded to the hydroxyl group, and tertiary alcohols have three carbon atoms bonded to the hydroxyl group.
How does alcohol structure influence physical properties such as boiling point and solubility?
Primary alcohols have higher boiling points and greater water solubility than secondary and tertiary alcohols due to stronger intermolecular hydrogen bonding.